|
|
Ions in solution
- Anion, cation (common names, formulas and charges for familiar ions; e.g., NH4+, ammonium; PO43-, phosphate; SO42-, sulfate)
| Common name | Formula |
| Anion |
| Hydroxide | OH- |
| Chloride | Cl- |
| Hypochlorite | ClO- |
| Chlorite | ClO2- |
| Chlorate | ClO3- |
| Perchlorate | ClO4- |
| Halide, hypohalide, etc | X-, XO-, etc |
| Carbonate | CO32- |
| Hydrogen Carbonate (Bicarbonate) | HCO3- |
| Sulfate | SO42- |
| Hydrogen Sulfate (Bisulfate) | HSO4- |
| Sulfite | SO32- |
| Thiosulfate | S2O32- |
| Nitrate | NO3- |
| Nitrite | NO2- |
| Phosphate | PO43- |
| Hydrogen Phosphate | HPO42- |
| Dihydrogen Phosphate | H2PO4- |
| Phosphite | PO33- |
| Cyanide | CN- |
| Thiocyanate | SCN- |
| Peroxide | O22- |
| Oxalate | C2O42- |
| Acetate | C2H3O2- |
| Chromate | CrO42- |
| Dichromate | Cr2O72- |
| Permanganate | MnO4- |
| Cation |
| Hydronium | H3O+ |
| Ammonium | NH4+ |
| Metal | Mn+ |
- Hydration, the hydronium ion
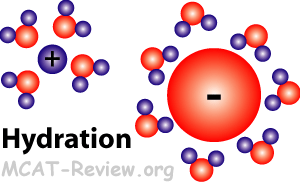
- Another name for hydration is solvation.
- Hydration is where water forms a shell around ions in solution.
- The oxygen atom on water is partially negative, so it surrounds cations.
- The hydrogen atoms on water is partially positive, so they surround anions.
- Hydronium ion = H3O+
- H+ never exist as a proton in water, it always exists as the hydronium ion.
Solubility
- Units of concentration (e.g., molarity)
- Molarity = M = mol solute/L solution
- Molality = m = mol solute/kg solvent
- Normality = N = Molarity of the species that matter.
- 1 M HCl = 1 N HCl
- 1 M H2SO4 = 2 N H2SO4
- 1 M H3PO4 = 3 N H3PO4
- x % = x g / 100 g = x g / 100 mL
- x ppm = x parts per million = x mg / kg = x mg / L
- Solubility product constant, the equilibrium expression
- Solubility product constant = Ksp
- AgCl (s) ↔ Ag+ (aq) + Cl- (aq)
- Ksp for AgCl = [Ag+][Cl-]
- Ag2SO4 (s) ↔ 2Ag+ (aq) + SO42- (aq)
- Ksp for Ag2SO4 = [Ag+]2[SO42-]
- Ksp values are found in a table:
- Ksp for AgCl = 1.8 x 10-10
- Ksp for Ag2SO4 = 1.2 x 10-5
- Ksp is simply Keq for dissolutions.
- The higher the Ksp, the more the reaction products dominate in a saturated solution (at equilibrium).
- What is the solubility of MX2 if given Ksp?
- MX2 ↔ M2+ + 2X-
- Ksp = [M2+][X-]2
- Ksp = [M2+][2M2+]2 (because for every M2+, there's two times as much X-)
- Ksp = 4[M2+]3
- Solve for [M2+]. Solubility is the same thing as [M2+] because you used Q = Ksp for a saturated solution.
- If you solved for [X-] instead, divide your results by 2.
- If you were given solubility and asked to solve Ksp, then know that solubility = [M2+] = [X-]/2
- Common-ion effect, its use in laboratory separations
- The common-ion effect is simply Le Chatelier's principle applied to Ksp reactions.
- AgCl (s) ↔ Ag+ (aq) + Cl- (aq)
- The common-ion effect says that if you add Cl- to the solution above, then less AgCl would dissolve.
- For example, if you add NaCl to a saturated solution of AgCl, then some AgCl will crash out of solution.
- Another example: more AgCl can dissolve in pure water than in water containing Cl- ions.
- In laboratory separations, you can use the common ion effect to selectively crashing out one component in a mixture.
- For example, if you want to separate AgCl from a mixture of AgCl and Ag2SO4, then you can do so by adding NaCl. This will selectively crash out AgCl by the common ion effect (Cl- being the common ion).
- Complex ion formation
- Metal+ + Lewis base: → Complex ion
- M+ + L → M-Ln+
- The Lewis base can be charged or uncharged.
- The Keq for this reaction is called Kf, or the formation constant.
- Complex ions and solubility
- The "complex ion effect" is the opposite of the common ion effect.
- AgCl (s) ↔ Ag+ (aq) + Cl- (aq); M+ + Cl- ↔ M-Cln complex ion.
- When complex ion forms, the Cl- ion is taken out, so more of the AgCl will dissolve.
- Alternatively: AgCl (s) ↔ Ag+ (aq) + Cl- (aq); NH3 + Ag+ ↔ Ag-(NH3)n complex ion.
- Here, the complex ion formation takes out Ag+, again causing more AgCl to dissolve.
- Solubility and pH
- Acids are more soluble in bases.
- HA → H+ + A-
- Putting the above in a base will take out the H+, thus, more HA will dissolve according to Le Chatelier's principle.
- Bases are more soluble in acids.
- B + H+ → BH+
- Putting the above in an acid will add more H+, and thus, drive more B to dissolve according to Le Chatelier's principle.
|
|
