|
|
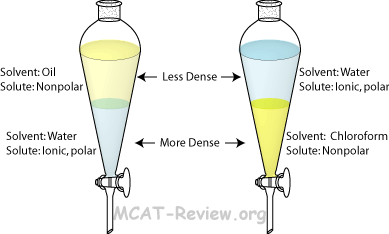
Extraction (Distribution of Solute Between Two Immiscible Solvents
|
Organic phase |
Aqueous phase |
| Solvent |
Nonpolar solvent |
Water |
| Solute |
Nonpolar solutes dissolve here |
Ionic and polar solutes dissolve here |
| Density |
The organic phase does not always float on top. Chloroform, for example, sinks below the aqueous phase. |
Water is usually denser than other solvents, but some organic solvents are even denser. |
Distillation
- Separates liquids based on boiling point. The stuff with the lower boiling point is boiled off and collected; the higher boiling point stuff remains behind.
- Simple distillation = done with a normal column = can separate two liquids if the difference in boiling point is large.
- Fractional distillation = done with a fractionating column = can separate two liquids with smaller differences in boiling point.
- Vacuum distillation = done under lower pressure (vacuum) = lowers the boiling point for all liquid components so you don't have to crank up the temperature so high (chemical might decompose).
Chromatography (Basic Principles Involved in Separation Process)
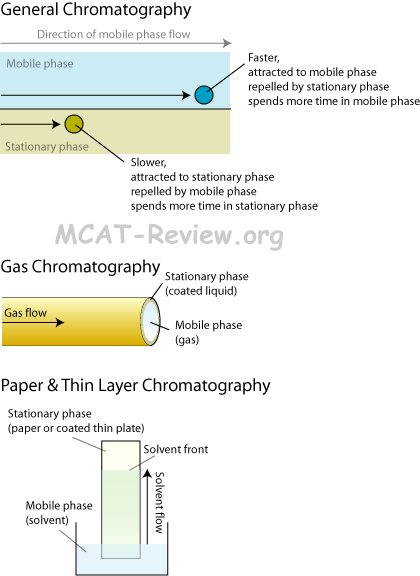
- Gas-liquid chromatography
- Good if analyte can be promoted to gas phase.
- Gas-liquid chromatography (GLC) is the same thing as gas chromatography (GC).
- The gas part is the mobile phase, the liquid part is the stationary phase coated to the inside walls of the column.
- Substrate equilibrates between mobile (gas) and stationary (liquid coat) phase.
- Those with greater affinity for the stationary phase comes out of the column slower. Polar substrate has more affinity for polar stationary phase, and hydrophobic substrate has more affinity for hydrophobic stationary phase.
- Paper chromatography
- Classically used to separate pigments in dyes.
- Solvent = mobile phase. Paper = stationary phase.
- Pigments in dyes stick to paper, solvent tries to wash them along, those with greater affinity to paper stays behind, those with greater affinity to solvent gets washed along.
- Rf value = distance traveled by pigment / distance of solvent front.
- Rf = 0 means that pigment has not moved.
- Rf = 1 means that pigment moved as far as the solvent front.
- Thin-layer chromatography
- Thin-layer chromatography = advanced paper chromatography.
- Instead of paper, you have a plate coated with a specific stationary phase of your choosing.
- Rf value used in the same way as paper chromatography.
Recrystalization (Solvent Choice from Solubility Data)
- Recrystalization = barely dissolving your compound, then let it recrystalize out of solution = compound ends up being more pure.
- Barely dissolving = use just enough to fully dissolve your compound under warm temperature = saturated solution.
- Recrystalize = solution cools, solubility decreases, compound comes out of solution.
- Solvent choice = choose a solvent in which your compound is soluble in at warm temperature, but not at cool temperature. Also, choose a solvent in which impurities are highly soluble.
- Impurities should remain dissolved in the solvent even when your compound recrystalizes out.
|
|
