|
|
Reaction rates
- The reaction rate is defined as the rate of change in the concentration of reactants or products. ie. how fast a reactant gets used up, and how fast a product gets produced.
- Rate = -ΔReactant/ΔTime = how fast a reactant disappears.
- Rate = ΔProduct/ΔTime = how fast a product forms.
- The unit for rate is molarity per second, or M/s.
Dependence of reaction rate upon concentration of reactants; rate law
- The rate law is the equation that describes the rate = the product of reactants raised to some exponents.
- aA + bB → cC + dD
- If the above reaction is single-step, then rate = k[A]a[B]b
- If the above reaction is the rate-determining step of a multi-step reaction, then the rate of the multi-step reaction = k[A]a[B]b
- If the above reaction is a multi-step reaction, then rate = k[A]x[B]y, where x and y are unknowns that correspond to the rate-determining step.
- To determine the rate law, you refer to a table of rates vs reactant concentrations.
-
| [A] (M) | [B] (M) | [C] (M) | rate (M/s) |
| 1 | 1 | 1 | 1 |
| 2 | 1 | 1 | 4 |
| 1 | 2 | 1 | 2 |
| 1 | 1 | 2 | 1 |
- r = k[A]x[B]y[C]z
- From this table, a 2x increase in [A] corresponds to a 4x increase in the rate. 2x = 4, so x = 2.
- A 2x increase in [B] corresponds to a 2x increase in the rate. 2y = 2, so y = 1.
- A 2x increase in [C] corresponds to 1x (no change) in rate. 2z = 1, so z = 0.
- r = k[A]2[B]1[C]0
- r = k[A]2[B]
- rate constant
- The k in the rate law is the rate constant.
- The rate constant is an empirically determined value that changes with different reactions and reaction conditions.
- reaction order
- Reaction order = sum of all exponents of the concentration variables in the rate law.
- Reaction order in A = the exponent of [A]
-
| Reaction Type | Reaction Order | Rate Law(s) |
| Unimolecular | 1 | r = k[A] |
| Bimolecular | 2 | r = k[A]2, r = k[A][B] |
| Termolecular | 3 | r = k[A]3, r = k[A]2[B], r = k[A][B][C] |
| Zero order reaction | 0 | r = k |
Rate determining step
- The slowest step of a multi-step reaction is the rate determining step.
- The rate of the whole reaction = the rate of the rate determining step.
- The rate law corresponds to the components of the rate determining step.
Dependence of reaction rate on temperature
- Activation energy
- Activated complex or transition state
- Activated complex = what's present at the transition state.
- In the transition state, bonds that are going to form are just beginning to form, and bonds that are going to break are just beginning to break.
- The transition state is the peak of the energy profile.
- The transition state can go either way, back to the reactants, or forward to form the products.
- You can't isolate the transition state. Don't confuse the transition state with a reaction intermediate, which is one that you can isolate.
- Interpretation of energy profiles showing energies of reactants and products, activation energy, ΔH for the reaction
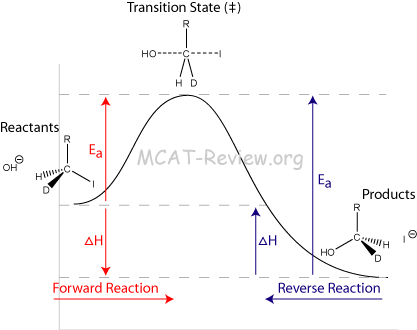
- The activation energy is the energy it takes to push the reactants up to the transition state.
- ΔH is the difference between the reactant H and the product H (net change in H for the reaction).
- H is heat of enthalpy.
- Exothermic reaction = negative ΔH
- Endothermic reaction = positive ΔH
- Arrhenius equation
- k = Ae-Ea/RT
- k is rate constant, Ea is activation energy, T is temperature (in Kelvins), R is universal gas constant, A is a constant.
- What this equation tells us: Low Ea, High T → large k → faster reaction.
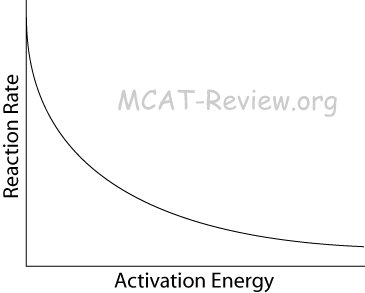
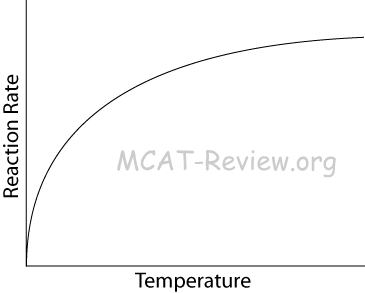
- When activation energy approaches zero, the reaction proceeds as fast as the molecules can move and collide.
- When temperature approaches absolute zero, reaction rate approaches zero because molecular motion approaches zero.
Kinetic control versus thermodynamic control of a reaction
- A reaction can have 2 possible products: kinetic vs thermodynamic product.
- Kinetic product = lower activation energy, formed preferentially at lower temperature.
- Thermodynamic product = lower (more favorable/negative) ΔG, formed preferentially at higher temperature.
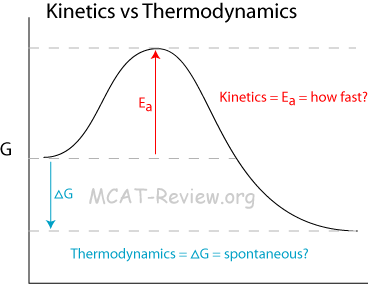
- Thermodynamics tells you whether a reaction will occur. In other words, whether it is spontaneous or not.
- A reaction will occur if ΔG is negative.
- ΔG = ΔH - TΔS
| Factors favoring a reaction | Factors disfavoring a reaction |
| Being exothermic (-ΔH) | Being endothermic (+ΔH) |
| Increase in entropy (positive ΔS) | Decrease in entropy (negative ΔS) |
| Temperature is a double-edged sword. High temperatures amplify the effect of the ΔS term, whether that is favoring the reaction (+ΔS) or disfavoring the reaction (-ΔS) |
- Kinetics tells you how fast a reaction will occur.
- A reaction will occur faster if it has a lower activation energy.
Catalysts; the special case of enzyme catalysis
- Catalysts speed up a reaction without getting itself used up.
- Enzymes are biological catalysts.
- Catalysts/enzymes act by lowering the activation energy, which speeds up both the forward and the reverse reaction.
- Catalysts/enzymes alter kinetics, not thermodynamics.
- Catalysts/enzymes help a system to achieve its equilibrium faster, but does not alter the position of the equilibrium.
- Catalysts/enzymes increase k (rate constant, kinetics), but does not alter Keq (equilibrium).
Equilibrium in reversible chemical reactions
- Law of Mass Action
- The Law of Mass Action is the basis for the equilibrium constant.
- What the Law of Mass Action says is basically, the rate of a reaction depends only on the concentration of the pertinent substances participating in the reaction.
- Using the law of mass action, you can derive the equilibrium constant by setting the forward reaction rate = reverse reaction rate, which is what happens at equilibrium.
- For the single-step reaction: aA + bB <--> cC + dD
- rforward = rreverse
- kforward[A]a[B]b = kreverse[C]c[D]d
- kforward/kreverse = [C]c[D]d/[A]a[B]b
- Keq = [C]c[D]d/[A]a[B]b
- This holds true for single and multi-step reactions, the MCAT will not ask you to prove why this is so.
- the equilibrium constant
- There are 2 ways of getting Keq
- From an equation, Keq = [C]c[D]d/[A]a[B]b
- From thermodynamics, ΔG° = -RT ln (Keq)
- Derivation: ΔG = 0 at equilibrium.
- ΔG = ΔG° + RT ln Q
- 0 = ΔG° + RT ln Qat equilibrium
- ΔG° = -RT ln Qat equilibrium
- At equilibrium:
- ΔG = 0
- rforward = rbackward
- Q = Keq
- Keq is a ratio of kforward over kbackward
- If Keq is much greater than 1 (For example if Keq = 103), then the position of equilibrium is to the right; more products are present at equilibrium.
- If Keq = 1, then the position of equilibrium is in the center, the amount of products is roughly equal to the amount of reactants at equilibrium.
- If Keq is much smaller than 1 (For example if Keq = 10-3), then the position of equilibrium is to the left; more reactants are present at equilibrium.
- The reaction quotient, Q, is the same as Keq except Q can be used for any point in the reaction, not just at the equilibrium.
- If Q < Keq, then the reaction is at a point where it is still moving to the right in order to reach equilibrium.
- If Q = Keq, the reaction is at equilibrium.
- If Q > Keq, then the reaction is too far right, and is moving back left in order to reach equilibrium.
- The reaction naturally seeks to reach its equilibrium
- application of LeChatelier's principle
- LeChatelier's principle: if you knock a system off its equilibrium, it will readjust itself to reachieve equilibrium.
- A reaction at equilibrium doesn't move forward or backward, but the application of LeChatlier's principle means that you can disrupt a reaction at equilibrium so that it will proceed forward or backward in order to restore the equilibrium.
-
| Reaction at equilibrium | What will induce the reaction to move forward | What will induce the reaction to move backward |
| A (aq) + B (aq) <--> C (aq) + D (aq) | Add A or B. Remove C or D. | Remove A or B. Add C or D. |
| A (s) + B (aq) <--> C (l) + D (aq) | Add B. Remove D. Adding or removing solids or liquids to a reaction at equilibrium doesn't do anything that will knock the system off its equilibrium. So, altering A and C won't make a difference. | Remove B. Add D. |
| A (s) + B (aq) <--> C (l) + D (g) | Add B. Remove D. Remove (decrease) pressure. | Remove B. Add D. Add (increase) pressure. |
| A (s) + B (g) <--> C (l) + D (g) | Add B. Remove D. Since both side of the balanced equation contains the same mols of gas products, modifying pressure is of no use. | Remove B. Add D. |
| A (s) + B (aq) <--> C (l) + D (aq) ΔH < 0 | Add B. Remove D. Removing heat by cooling the reaction. | Remove B. Add D. Add heat by heating the reaction. |
Relationship of the equilibrium constant and standard free energy change
- ΔG = ΔG° + RT ln Q
- Set ΔG = 0 at equilibrium.
- Q becomes Keq at equilibrium.
- 0 = ΔG° + RT ln (Keq)
- ΔG° = -RT ln (Keq)
|
|
