|
|

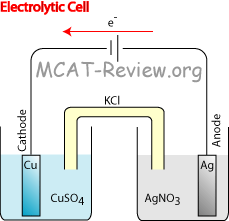

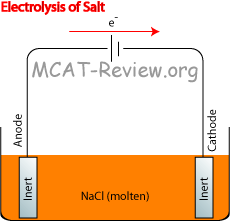

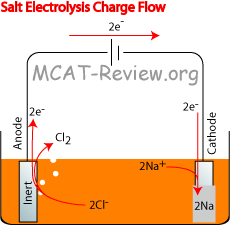
Electrolytic cell
- electrolysis
- Requires potential/voltage input. On the diagram, this is represented by a battery in the circuit. In contrast, a galvanic cell has in its place either a resistor, or a Voltmeter.
- The potential/voltage input + the cell potential must be > 0 for the reactions to occur.
- For electrolytic cells, the cell potential is negative, so a potential input greater than the magnitude of the cell potential must be present for electrolysis to occur.
- In contrast, galvanic/voltaic cells already have a positive cell potential. Thus, no input is required for galvanic/voltaic cells.
- In the diagram above, arrows are shown in red because the battery is forcing the flow of electrons. Normally, the electrons would want to flow the other way (or not flow at all).
- anode, cathode
- The following rules hold true for both electrolytic and galvanic/voltaic cells.
- Anode is always the place where oxidation happens.
- Cathode is always the place where reduction happens.
- Mnemonic:
- An Ox = ANode OXidation
- Red Cat = REDuction CAThode
- Anode shoots out electrons, Cathode takes in electrons.
- electrolyte
- Ions = electrolyte.
- Electrolytes conduct electricity by the motion of ions.
- Without electrolytes, there won't be a circuit because electricity won't be able to travel.
- Faraday's law relating amount of elements deposited (or gas liberated) at an electrode to current
- Current = coulombs of charge per second. I = q/t
- Faraday's constant = coulombs of charge per mol of electron = total charge over total mols of electrons. F = q/n.
- q = It and q = nF, thus we get:
- It = nF
- Current x time = mols of e- x Faraday's constant.
- Using this equation, you can solve for n, mols of electrons. Then using the half equation stoichiometry, you can find out how many mols of element is made for every e- transferred. For example, 1 mol of Cu is deposited for every 2 mols of electrons for the following half reaction: Cu2+ + 2e- → Cu.
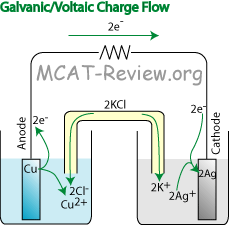
- electron flow; oxidation, and reduction at the electrodes
- Electrons shoot out of the anode because oxidation occurs there to lose electrons. M → M+ + e-.
- Electrons travel into the cathode, where it crashes into the cations on the surface of the cathode. This is because reduction occurs at the cathode to receive electrons. M+ + e- → M.
- Mnemonic:
- Oil Rig : Oxidation Is Losing e- Reduction Is Gaining e-.
- Oxidation is an increase in charge, Reduction is a decrease in charge.
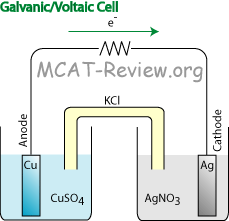
Galvanic or voltaic cell
- half reactions
- Oxidation half reaction describes the species that loses electrons (increases in charge). For example, Cu → Cu2+ + 2e-
- Reduction half reaction decribes the species that gains electrons (decreases in charge). For example, 2Ag+ + 2e- → 2Ag
- reduction potentials; cell potential
- You find reduction potentials in a table:
-
| Half reactions | Reduction potential (V) | comments |
| Cl2 + 2e- → 2Cl- |
+1.359 |
Chlorine has high electron affinity, it loves to gain electrons and being reduced. Thus, it has a high reduction potential. Similarly, species like oxygen, halogens, and nonreactive metals have positive reduction potentials. |
| 2H+ + 2e- → H2 |
0.000 |
Hydrogen is set to have a standard reduction potential of zero |
| Na+ + e- → Na |
-2.714 |
Sodium hates its electron, it gets rid of it to obtain a full outer shell and be stable as a cation. It is very hard to force electrons onto the stable cation to reduce it. Thus, it has a very negative reduction potential. Similarly, species like potassium and other reactive metals have negative reduction potentials. |
- Reduction potential = potential of the reduction half reaction.
- Oxidation potential = potential of the oxidation half reaction = reverse the sign of the reduction potential.
- Cell potential = Reduction potential + Oxidation potential.
- For example, the cell potential for the galvanic cell shown in the diagram is:
-
| Reduction potential table |
| Species | Reduction Potential (V) |
| Ag(I) | +0.799 |
| Cu(II) | +0.337 |
- Reduction half reaction: 2Ag+ + 2e- → 2Ag
- Reduction potential = +0.799
- Oxidation half reaction: Cu → Cu2+ + 2e-
- Oxidation potential = +0.337 x -1 = -0.337
- Cell potential = 0.799 - 0.337 = 0.462 V
- The cell potential for all galvanic/voltaic cells is positive, because the voltaic cell generates potential.
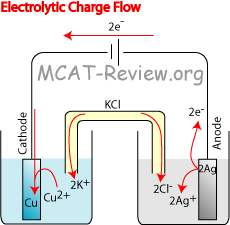
- Another example, the cell potential for the electrolytic cell shown in the diagram is:
- Reduction half reaction: Cu2+ + 2e- → Cu
- Reduction potential = +0.337
- Oxidation half reaction: 2Ag → 2Ag+ + 2e-
- Oxidation potential = +0.799 x -1
- Cell potential = 0.337 - 0.799 = -0.462 V
- The cell potential for all electrolytic cells is negative, because the electrolytic cell requires potential input.
- direction of electron flow
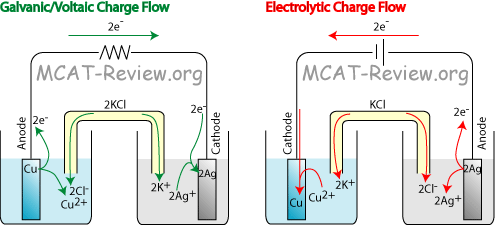
- Electrons always flow from the Anode to the Cathode. Mnemonic: A to C in alphabetical order. Or, think about AC power - the A comes first and stands for anode)
- Oxidation (at the anode) produces electrons (and cations), and shoots out the electrons toward the cathode. The cathode receives those electrons and uses them for reduction.
- Naturally, the species with the highest oxidation potential (lowest reduction potential) will be the anode, and the species with the highest reduction potential will be the cathode.
- In the diagram above, the Galvanic/Voltaic cell shows a natural flow because Cu (higher oxidation potential/lower reduction potential) is the anode, and Ag (higher reduction potential) is the cathode.
- However, the electrolytic cell shows exactly the opposite. In order to force the Cu to be the cathode and Ag to be the anode, a battery is used to drive the reaction.
- Electrons flow in wires and electrodes, while ions flow in the electrolyte solution, thus creating a completed circuit.
|
|
