|
|
Description
- nomenclature and classification, common names
- nomenclature
- Carbohydrate = Sugars, monosaccharides, disaccharides, polysaccharides
- Prefix:
- Deoxy = it has an -H in place of an -OH at a certain position.
- D/L = absolute configuration = assigned based on the chirality of the carbon atom furthest from the carbonyl group.
- α/β = anomeric configuration.
- Suffix: all sugars end in -ose.
- classification
- aldose = sugars with an aldehyde group.
- ketose = sugars with a ketone group.
- pyranose = sugars in a 6 membered ring structure = hexagon shaped. For example, glucopyranose = glucose in a 6 membered ring.
- furanose = sugars in a 5 membered ring structure = pentagon shaped. For example, fructofuranose = fructose in a 5 membered ring.
- #ose = sugar with # carbon atoms. For example, hexose = sugar with 6 carbons. Another example: aldopentose = a five-carbon sugar with an aldehyde group.
- In order to be classified as a carbohydrate, a molecule must have:
- at least a 3 carbon backbone.
- an aldehyde or ketone group.
- at least 2 hydroxyl groups.
- common names
- Common names are used so you can say glucose instead of (2R,3S,4R,5R)-2,3,4,5,6-Pentahydroxyhexanal
- See monosaccharides, disaccharides and polysaccharides
- absolute configuration
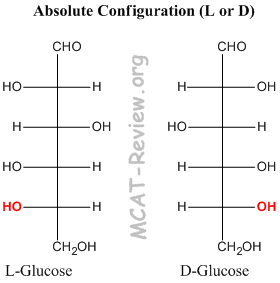
- The chiral carbon furthest from the carbonyl group determines the absolute configuration L or D of the sugar.
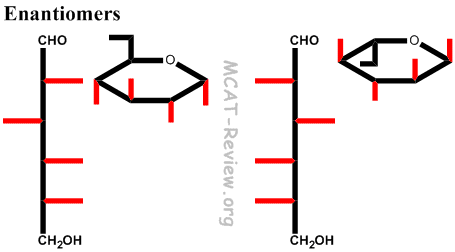
- If in the fischer projection, the OH group on the chiral carbon furthest from the carbonyl is pointing left, then it's L. If it's pointing right, then it's D.
- Note: L and D are enantiomers, not epimers. So, every chiral carbon center inverts. It's just that you assign L and D based on the chiral carbon furthest from the carbonyl.
- cyclic structure and conformations of hexoses
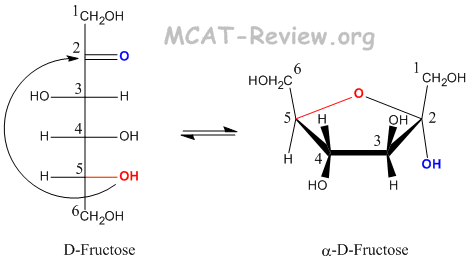
- Fructose forms a furanose when carbon 5 attacks the carbonyl carbon.
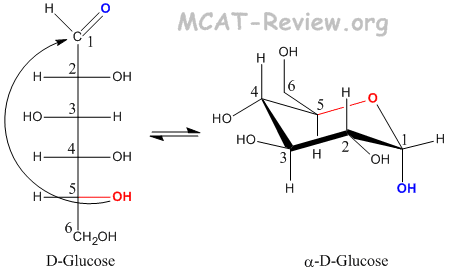
- Glucose forms a pyranose when carbon 5 attacks the carbonyl carbon.
- Convert a Fischer projection to Haworth (cyclic) form
- -OH groups that are pointing Left on the Fischer becomes Up on the Haworth.
- -OH groups that are pointing Right on the Fischer becomes Down on the Haworth.
- The -OH group on the anomeric carbon (the Fischer carbonyl) can be either up (beta) or down (alpha).
- The CH2OH group on the absolute configuration carbon (carbon 5) points up for D, and down for L.
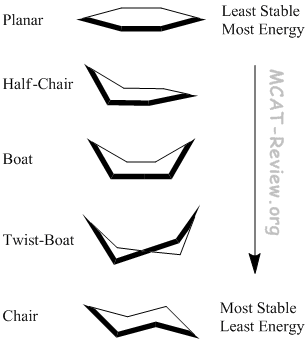
- In the planar conformation, everything is eclipsed.
- In the chair conformation, everything is staggered.
- All the conformations in between are partially eclipsed.
- The Boat conformation has Flagpole interactions because axial groups attached to the head and tail of the boat clash.
- The Twist-boat conformation lessens these Flagpole interactions in addition to reducing the number of eclipsed interactions.
- epimers and anomers
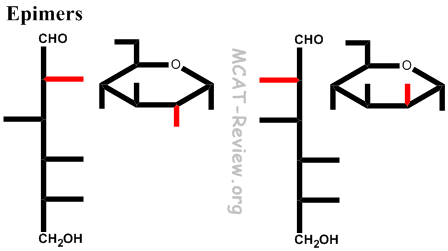
- Epimers = different configuration in just one chiral carbon.
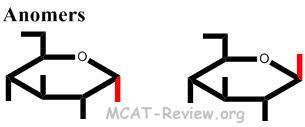
- Anomers = different configuration in the chiral, anomeric carbon when the molecule is in the cyclic form.
- Anomers are simply special types of epimers.
- Epimers are simply special types of diastereomers.
- Don't confuse with enantiomers (D/L configuration), in which everything changes configuration.
Hydrolysis of the glycoside linkage
- Glycoside linkage = acetal linkage = linkage involving the hydroxyl group of the anomeric carbon.
- Glycoside linkage can also mean the linkage between the sugar and the base in nucleotides.
- Examples of glycosidic linkages = starch, glycogen, nucleotide.
- Hydrolysis of the glycosidic bond has the same mechanism as hydrolysis of the acetal bond.
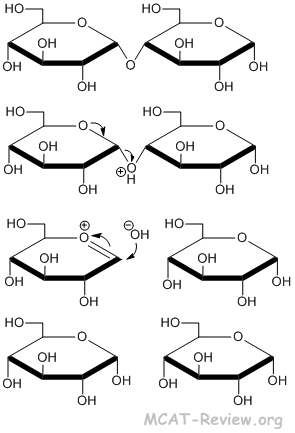
- glycoside + H2O + catalyst → hydrolysis.
- Catalysts include: Amylase for starch and glycosylase for nucleotide.
Monosaccharides
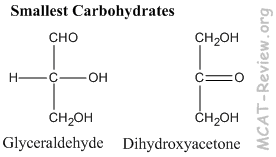
- The simplest, smallest carbohydrates are glyceraldehyde and dihydroxyacetone.
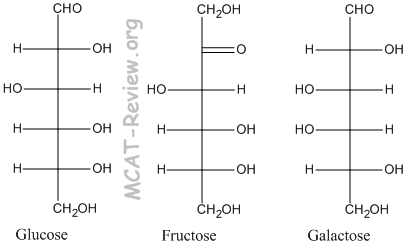
- The 3 common monosaccharides are glucose, fructose, and galactose. Glucose is our blood sugar and the product of photosynthesis. Fructose is the sugar in fruits, and it is sweeter than glucose. Galactose is one of the monomers that make up lactose, which is the sugar in milk; it is less sweet than glucose.
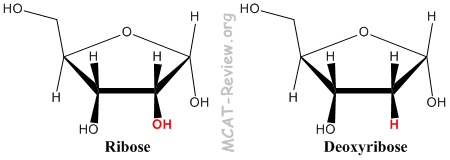
- The sugar that make up RNA is ribose, and for DNA it is deoxyribose (More precisely it's 2'-deoxyribose because the difference is at the 2 carbon).
Disaccharides
- Sucrose is a disaccharide made from α-glucose and β-fructose joined at the hydroxyl groups on the anomeric carbons (making acetals). Sucrose is table sugar, the sugar we buy in stores.
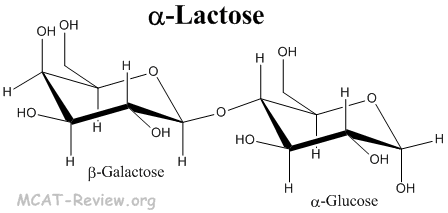
- Lactose is a disaccharide made from β-galactose and α/β-glucose joined by a 1-4 linkage.
Polysaccharides
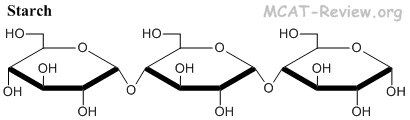
- Starch = glucose molecules joined by α1-4 linkage = energy storage in plants
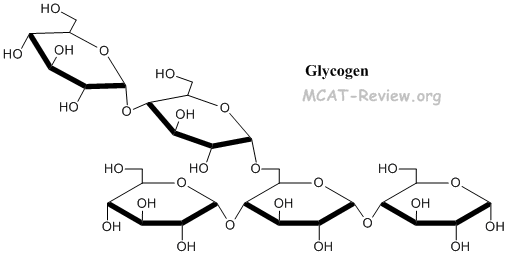
- Glycogen = same as starch, but with additional α1-6 linkages for branching = energy storage in animals (stored in the liver)
Reactions of Monosaccharides
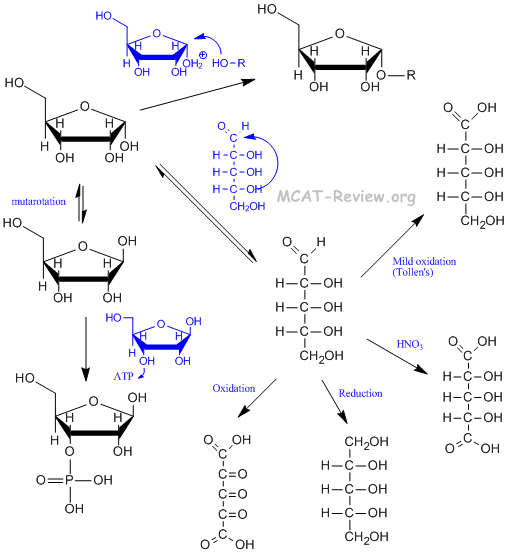
- Hemiacetal formation = -OH attacks carbonyl group = produces ring form.
- Acetal formation = another -OH attack on the same carbonyl group = produces polysaccharides if the -OH is from another monosaccharide.
- Mutarotation = equilibrium between the α and β anomers.
- Strong oxidation turns aldehyde and terminal hydroxyls to carboxylic acids, and other hydroxyls to ketones. The strongest kind of oxidation turns everything to CO2, and this occurs in cellular respiration.
- Mild oxidation is more selective. Tollens agent (the test for aldoses, silver reagent) selectively oxidizes the aldehyde to carboxylic acid. Nitric acid oxidizes both the aldehyde and the terminal hydroxyl to carboxylic acids, but leaves the other hydroxyls alone.
- Reduction turns monosaccharides into polyalcohols.
|
|
