|
|
The ionic bond (electrostatic forces between ions)
- The ionic bond forms when electrons transfer completely from one atom to another, resulting in oppositely charge species that attract each other via electrostatic interaction.
- Electrostatic energy α q1q2/r
- Electrostatic Energy = Electrostatic potential x charge = kq1/r x q2 = kq1q2/r
- Electrostatic energy is negative because q1 and q2 are opposite in charge (If q1 and q2 are not opposite in charge, then they would repel each other, and no ionic bond would form).
- Frequently, the negative sign is dropped and only the magnitude of the electrostatic energy is used.
- The greater the magnitude of electrostatic potential, the stronger the ionic bond.
- Strong ionic bonds are promoted by high charge magnitudes (q values) that are close together (small r value).
- Ions that form strong ionic bonds have high charge density, that is, the charge to size ratio is high.
- Electrostatic energy α lattice energy
- Lattice energy measures the ionic bond strength.
- Lattice energy is the energy required to break the ionic bond.
- The larger magnitude of the lattice energy, the stronger the ionic bond and the harder it is to break.
- The lattice energy is proportional to the electrostatic attraction between the ions.
- Electrostatic force α q1q2/r2
- Coulomb's law: F = kq1q2/r2
- Larger charge magnitudes + charges being closer together → greater electrostatic force.
- The Coulomb's constant, k, is 9E9.
- Opposite charges attract (negative F), same charges repel (positive F).
- If q1 doubles, the electrostatic force doubles.
- If r halves, the electrostatic force increase by a factor of 22 = 4.
- Coulomb's law is analogous to the universal law of gravitation:
- F = Gm1m2/r2
- G is analogous to k and m is analogous to q.
- The big difference is that G is tiny compared to k, because gravitational force is weaker compared to the much stronger electrostatic force.
The covalent bond
- The covalent bond results when there is a sharing of electrons between two atoms, resulting in the overlap of their electron orbitals.
sigma and pi bonds
- σ bonds are single bonds. They also make up the first bond of double and triple bonds.
- π bonds are double and triple bonds. They make up the second bond in a double bond, and both the second and the third bond in a triple bond.
hybrid orbitals: sp3, sp2, sp and respective geometries
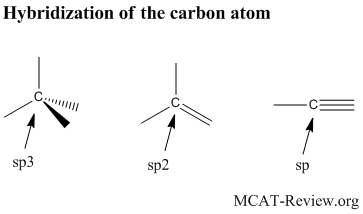
- Hybrid orbitals are produced by hybridizing (mixing) electron orbitals to produce geometries that facilitate bonding.
- Sp3: a hybrid between one s with 3 p orbitals. Tetrahedral in geometry. Contains single bonds only.
- Sp2: a hybrid between one s with 2 p orbitals. Trigonal planar in geometry. Contains a double bond.
- Sp: a hybrid between one s with one p orbital. Linear in geometry. Contains a triple bond.
- Hybrid orbitals are most commonly used with carbon as the center atom.
valence shell electron pair repulsion and the prediction of shapes of molecules (e.g., NH3, H2O, CO2)
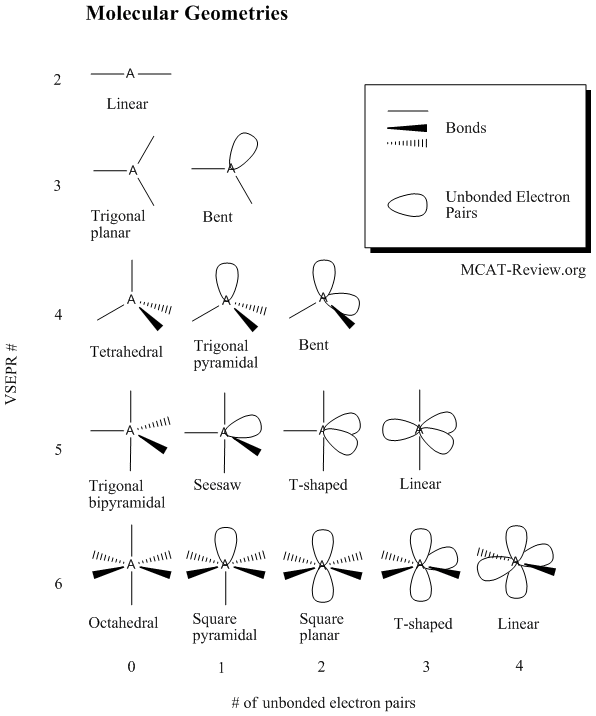
- In short, it is the VSEPR theory.
- The VSEPR theory is used to predict the geometry of molecules.
- The shapes of molecules are determined by the molecular geometry.
- Radicals also count as an electron pair.
- The VSEPR number is the total number of bonds + unbonded electron pairs.
- When calculating the VSEPR number, always use the electron/bond configuration about the central atom.
- NH3 has a vsepr number of 4 (3 bonds to H and 1 unbonded pair). If you look up the table for VSEPR # = 4 and # unbonded electron pairs = 1, then you'll find that NH3 is trigonal pyramidal.
- H2O has 2 bonds, 2 unbonded electron pairs - it is bent.
- CO2 has 2 double bonds and 0 unbonded electron pairs - it is linear.
Lewis electron dot formulas
- Every dot represents 1 electron. Every line represents 1 bond (2 electrons). A "lone pair" is represented by two dots.
- Formulas are drawn in such a way that an octet is achieved on each atom. Exceptions include the boron column (they form 3 bonds and have a six-tet), large elements (3rd row and below such as the 10-tet P in PO43- and the 12-tet S in SO42-), and radicals (compounds with an odd # total electrons that result in a single, unpaired electron).
- All electrons in a bond are shared and can be used to satisfy the octet for both atoms on either side of the bond.
- Rules of thumb for Lewis structures
- Carbon: 4 bonds total (meaning 4 total bonds. It can either be 4 single bonds or two double bonds ...etc) and no lone pairs. eg. CH4, CO2
- Oxygen: O can be
- O: 2 bonds total, 2 lone pairs. eg. H2O, O2
- O1-: 1 bond, 3 lone pairs, formal charge of -1.
- O1+: 3 bonds, 1 lone pair, formal charge of +1.
- Nitrogen: N can be
- N: 3 bonds total, 1 lone pair. eg. Amine or ammonia NH3
- N+: 4 bonds, 0 lone pair, formal charge of +1. eg. Ammonium NH4+
- Halogens: 1 bond, 3 lone pairs. eg. CCl4
- Hydrogen: 1 bond, 0 lone pair (exception to octet rule).
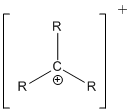
- Carbocation: C+ has 3 bonds, no lone pairs, formal charge +1.
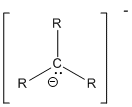
- Carbanion: C- has 3 bonds, 1 lone pair, formal charge -1.
- Boron: 3 bonds, 0 lone pairs (exception to the octet rule). eg. BH3
- Common Lewis structures
- Hydrogen Lewis structures
- Hydrogen Proton:

- Hydride ion:
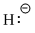
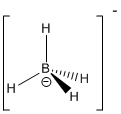
- Boron Lewis structures
- Borane:
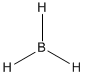
- Borohydride ion:
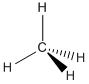
- Carbon Lewis structures
- Methane:
- Carbocation:
- Carbanion:
- Nitrogen Lewis structures
- Amine / Ammonia:
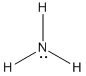
- Ammonium:
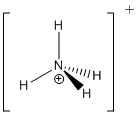
- Imine:
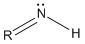
- Oxygen Lewis structures
- Molecular oxygen:
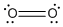
- Water, alcohol, and ethers:
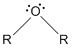
- Ozone:
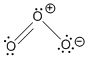
- Halogen Lewis structures
- Hydrogen fluoride:
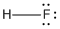
- Chloromethane:
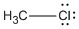
- Bromide ion:
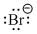
- R in the figures are either carbon or hydrogen.
- Lewis structures for elements in the same column (group) of the periodic table are similar to one another. For example, sulfur can be substituted for oxygen in lewis structures of oxygen.
resonance structures
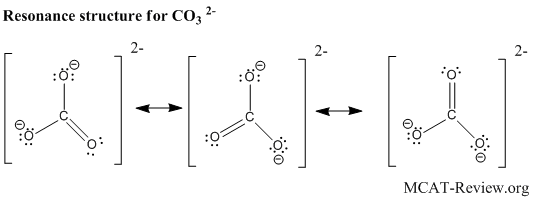
- When there are more than 1 satisfactory Lewis structures for a molecule, they are called resonance structures.
- You can visualize the molecule "shifts" between each of its resonance structures really fast, spending more time in the more stable resonance structures. Or more accurately, the structure of the molecule is a "combination" of its resonance structures, taking on more character from the most stable resonance structures. Eg. The bond length of a molecule that has both a single and a double bond resonance structure is intermediate between a single bond and a double bond.
- The molecule spends most of its time in the most stable resonance structure.
- Stable properties:
- Octet rule is satisfied in every atom (except for boron group and hydrogen).
- No formal charges.
- If there must be formal charges, like charges are apart and unlike charges are close together.
formal charge
- Formal charge = valence electron # in the unbonded atom - electron # in the bonded atom.
- Electron # in the bonded atom = dots around the atom + lines connected to the atom.
- The dots around the atom represent electrons that are held entirely by the atom.
- The lines connected to the atom represent bonding electron pairs, in which the atom only gets one of the two electrons.
- Formal charges (other than 0) must be labeled next to the atom with the formal charge.
- Common formal charges:
- Oxygen with only a single bond: -1.
- Oxygen with no bond but have an octet: -2. (Oxygen usually exists as the diatomic O2 and have a double bond to themselves)
- Carbon with only 3 bonds: either +1 if carbocation or -1 if carbanion.
- Nitrogen with 4 bonds: +1.
- Halogen with no bonds, but have an octet: -1. (Halogens usually exist as a diatomic and have a single bond to themselves such as Cl2)
- Boron with 4 bonds: -1. eg. BH4-
Lewis acids and bases
- Lewis acid accept electron pairs. They don't have lone pairs on the central atom. eg. BF3
- Lewis bases donate electron pairs. They have lone pairs on their central atom. eg. NH3
Partial ionic character
- Covalent bonds between atoms with dissimilar electronegativities have a partial ionic character.
- role of electronegativity in determining charge distribution
- The more electronegative atom receives a partial negative charge.
- The less electronegative atom receives a partial positive charge.
- dipole moment
- Molecules with asymmetrical partial charge distribution have a dipole moment. eg. H2O has a dipole moment because the molecule is bent and the oxygen-side of the molecule is partially negative.
- Dipole moment depends on charge and distance.
- The greater electronegativity difference, the greater the charge and hence the dipole moment.
- The greater the distance separating the charges, the greater the dipole moment.
- Molecules with symmetrical partial charge distribution do not have dipole moments. eg. CCl4 do not have a dipole moment because the partially negative chlorine atoms are arranged symmetrically in a tetrahedron. The symmetry cancels out their individual dipole moments.
- Things with a dipole moment are said to be polar.
- Are the individual bonds in CCl4 polar? Ans: yes.
- Is the entire molecule CCl4 polar? Ans: no.
Old topics
The topics below are outdated. They have been either modified or replaced by the most recent aamc publication.
- E = kQ1Q2/d
- Energy = Electrostatic potential x charge = kQ1/d x Q2 = kQ1Q2/d
- E is negative because Q1 and Q2 are opposite in charge.
- The more negative E is, the stronger the ionic bond.
- Strong ionic bonds are promoted by high charge magnitudes (Q values) that are close together (small d value).
- E = lattice energy
- The name used for E is the lattice energy, and it measures the ionic bond strength.
- Lattice energy is the energy required to break the ionic bond.
- The larger magnitude of the lattice energy, the stronger the ionic bond and the harder it is to break.
- Force attraction = R(n+e)(n-e)/d^2
- The above equation describes the force of attraction between the cation n+ and the anion n- at a distance d apart.
- R is Coulomb's constant (usually written as k).
- n+e = charge of cation in coulombs = positive charge (n+) times coulombs per electron (e).
- n-e = charge of anion in coulombs = negative charge (n-) times coulombs per electron (e).
- The elementary charge or coulombs per electron (e) is 1.6E-19, but you don't have to memorize it. The MCAT will give it to you.
- The Coulomb's constant is 9E9.
- The official Coulomb's law states: F = kQ1Q2/r2
|
|
