|
|
Description
- nomenclature:
|
suffix |
example |
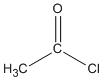
| Acid chlorides |
-oyl chloride |
ethanoyl chloride
 |
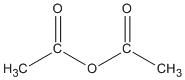
| Anhydrides |
-oic anhydride |
ethanoic anhydride
 |
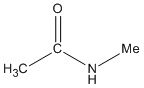
| Amides |
-amide |
N-methyl ethanamide
 |
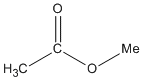
| Esters |
-oate |
methyl ethanoate
 |
- physical properties
- C=O bond is polar, so there are dipole-dipole interactions.
- No hydrogen bond exists in acid chlorides, anhydrides, or esters unless there is an -OH group somewhere.
- Amides can hydrogen bond because of the N-H group. In fact, hydrogen bonding involving the amide backbone of polypeptides form the secondary structure of proteins.
- Amides have higher boiling points than the other acid derivatives.
- Acid derivatives have high boiling points than alkanes because of the C=O dipole interactions.
- infrared absorption
- Acid chloride: the C=O will show up at greater than 1700 cm-1, pretty close to 1800 cm-1
- Anhydride: the double C=O doesn't show up as a single band. Instead, 2 bands shows up between 1700 cm-1 and 1800 cm-1.
- Amide: the N-H shows up around 3300 cm-1, the C=O shows up at 1700 cm-1
- Ester: C=O group shows up at 1700 cm-1. The C-O ether stretch shows up around 1200 cm-1
Important reactions
- preparation of acid derivatives
- Carboxylic acid + SOCl2 → Acid chloride.
- Carboxylic acid + carboxylic acid + heat → Anhydride.
- Acid chloride + carboxylic acid + base → Anhydride.
- Acid chloride + alcohol + base → Ester.
- Acid chloride + amine → Amide.
- Acid chloride + water → Carboxylic acid.
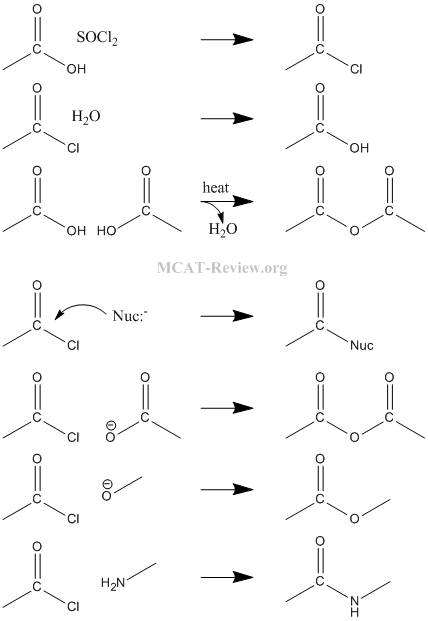
- nucleophilic substitution: Nucleophile attacks the carbon center of the C=O group.
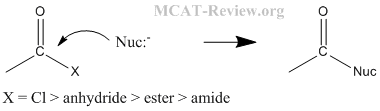
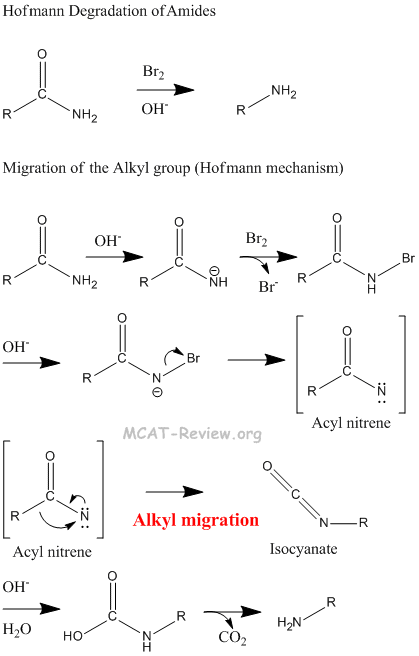
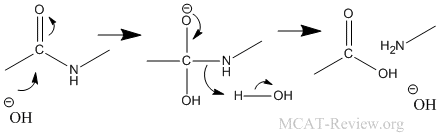
- Hofmann rearrangement: Hofmann rearrangement takes away the C=O of an amide. The alkyl migration is basically how the -R group on the other side of the C=O migrates and attaches itself to the nitrogen atom. See figure below for detailed mechanism of the Hofmann degradation and how the aryl group migrates.
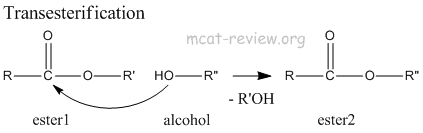
- transesterification: Ester + alcohol → new ester.
- hydrolysis of fats and glycerides (saponification): saponification is basically the hydrolysis of an ester in base.
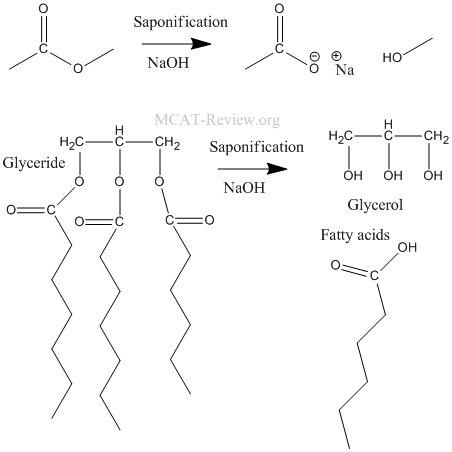
- hydrolysis of amides: the leaving group is not NR2-, it is the neutral amine.
General principles
- relative reactivity of acid derivatives: Acid chloride > Anhydride > Esters > Amides
- Acid halides are the most reactive derivatives because halides are very good leaving groups.
- Amides are the most stable derivatives because NR2- is a terrible leaving group. Also, the C-N bond has a partial double bond characteristic. Proteins are made of peptide bonds, and they are very stable.
- steric effects: bulky groups around the C=O group helps protect the carbon center from nucleophilic attack.
- electronic effects: groups that can redistribute and stabilize negative charges are good leaving groups. For example, the anhydride has a good leaving group - the carboxylate ion - because the COO- can redistribute the negative charge to both oxygens via resonance.
- strain (e.g., beta-lactams)
- Amides have a double bond characteristic between the carbon and nitrogen. This means that the C-N bond can not rotate.
- Normally, the sigma bonds in a ring rotate as to achieve the most stable conformation, but this can't occur for the C-N bond if the ring contains an amide.
- Because C-N bond in an amide can not rotate, rings that contain amides have higher strain.
- An example of this is the beta-lactam, which is basically a 4 membered ring with 1 amide in it.
|
|
